Lewis Lead Parallax
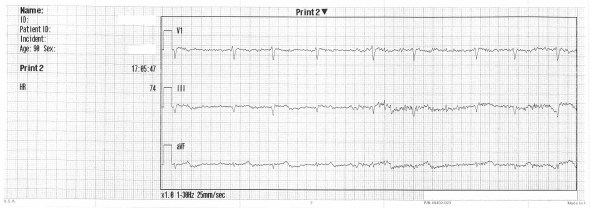
The following was recorded from a 90 year-old Caucasian man with shortness of breath.
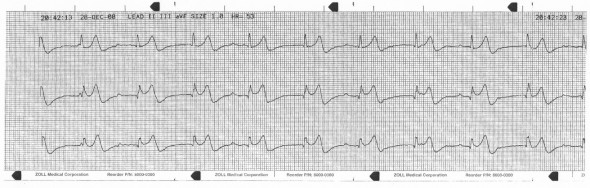
This is very suspicious for second degree block; bigeminal PACs or PJCs with compensatory pauses are also possibilities. There is poor visualization of atrial activity due to background noise and intrinsic low voltage. The 12-lead is referenced for the best lead to visualize:

V1 seems promising; a V1 rhythm strip is acquired:
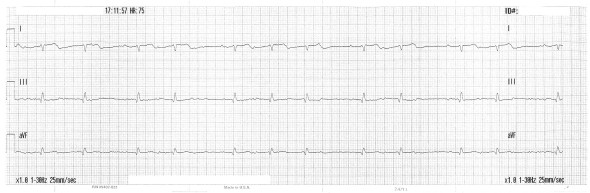
Worse still. Next, the Lewis lead:
Now the diagnosis is transparent. Before resorting to the placement of Lewis electrodes, the voltage gain can be doubled and noise reduction strategies applied. These were not utilized here.
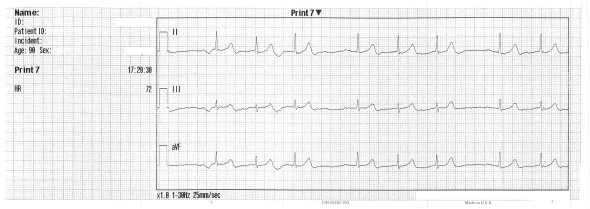
12-lead under Lewis electrode placement:
There is diminished QRS voltage, new inferior Q-waves and ST-segment abnormalities. This could easily be misinterpreted, but it is an artifact of the lead system.
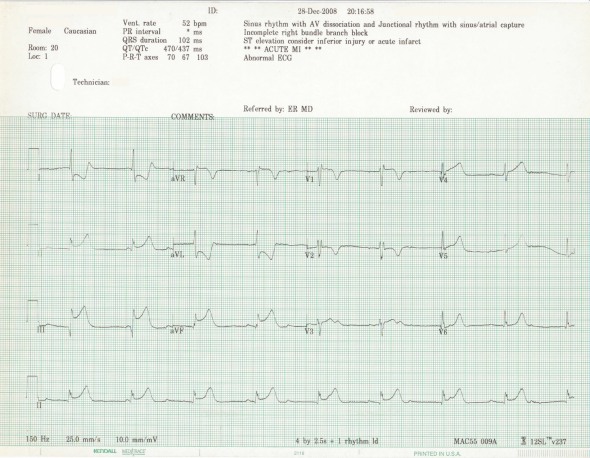
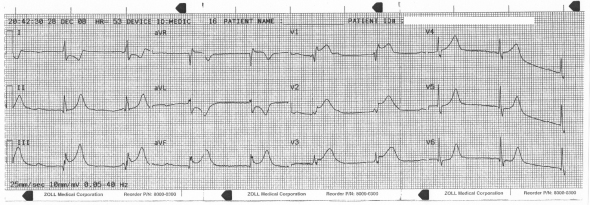
Switching back to standard placement. Different electrodes were used at this time and the voltage is altered; the background noise has faded but the atria remain quiet:
This patient was ultimately found to be pancytopenic (WBCs 3.1, RBCs 1.17, Hem 4.8, Crit 13.0, Platelets 96, Neu 23, Lym 60, Monos 16) and was worked up for myelodysplastic syndrome. The electrocardiographic findings may be associated with the anemia; they may also be incidental.
Christopher Watford brought the Lewis lead to my attention; he has described its physiology and advantages extensively in his blog post, Highlighting Atrial Activity on an ECG: The S5 Lead, as well as via audio on the EMCrit Podcast with Scott Wiengart.
There are numerous alternative lead systems: Brughada leads, high and low precordial placements for visualizing poorly represented territories, systems designed to emphasize pacemaker activity, etc. Body surface mapping technologies (e.g. The 80-Lead Prime ECG) have also shown promise. Some of these lead systems are described on this site under EKG Resources.
References
Bakker, A., et al. (2009). The lewis lead: Making recognition of P waves easy during wide QRS tachycardia. Circulation, (2009), 119; e592-e593. [Free Full Text] doi: 10.1161/CIRCULATIONAHA.109.852053
Lewis T. (1931). Auricular fibrillation. Clinical Electrocardiography. 5th ed. London, UK: Shaw and Sons; 1931: 87–100.
The Persistence of Memory: A return to “Atypical symptoms with a critical lesion.”
In 2010, as part of a case series exploring the presentation of AV block in the setting of inferior wall STEMI, I discussed the following EKGs:
It was hypothesized from these tracings that a proximal RCA lesion was responsible for the manifest inferior wall and right ventricular involvement, likely in the setting of a right dominant coronary system owing to the AV nodal dysfunction.
Recently I was able to follow up on this case.
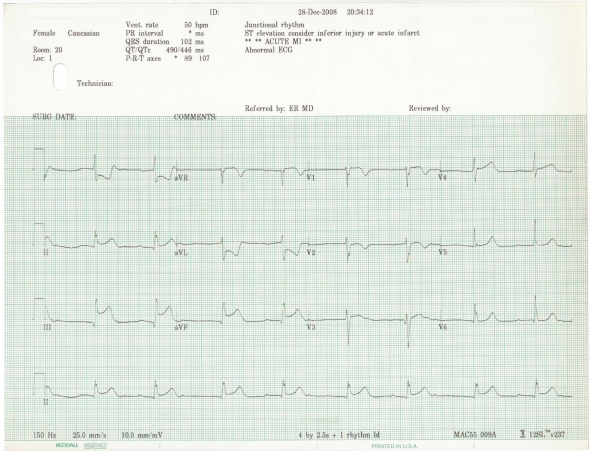
This was a 64 year-old Caucasian female complaining of nausea, vomiting, and a syncopal episode while attempting to ambulate. Her history was significant for HTN, hyperlipidemia, breast cancer, and a mechanical aortic valve replacement in 2005. At that time a presurgical cath had identified a 70% ostial RCA stenosis. This baseline EKG was recorded on admission:
 In 2008, on the date the case study EKGs were recorded, she became progressively hypotensive while in the emergency department and required intubation and vasopressor circulatory support. During emergent catheterization stents were placed across a 99% diffuse ostial RCA lesion and a further stenosis of the distal RCA. The interventionalist described a right dominant coronary system with a small LAD and circumflex. On arrival in the ED, a troponin of 0.14ng/mL was recorded. At noon on the following day the value had climbed to 55.20 and peaked at over 90 later that evening.
In 2008, on the date the case study EKGs were recorded, she became progressively hypotensive while in the emergency department and required intubation and vasopressor circulatory support. During emergent catheterization stents were placed across a 99% diffuse ostial RCA lesion and a further stenosis of the distal RCA. The interventionalist described a right dominant coronary system with a small LAD and circumflex. On arrival in the ED, a troponin of 0.14ng/mL was recorded. At noon on the following day the value had climbed to 55.20 and peaked at over 90 later that evening.
Due to cardiogenic shock, both an IABP and temporary pacemaker were placed. A long ICU course ensued during which numerous abnormalities were identified and addressed including but not limited to severe sepsis, a ruptured breast implant, gall bladder disease, elevated LFTs, and a renal mass suspicious for carcinoma. The following EKGs were recorded on ICU days 2, 3, and 4.
In June of 2009 she was again admitted to the hospital, at this time with a primary diagnosis of aspirational pneumonia complicated by renal insufficiency.
A tracheotomy was placed. Respiratory failure and sepsis were treated in the ICU and ventilator weaning was begun only to rebound with recurrent episodes of septic shock. This was repeated four times over a two-month period. Renal function progressively declined, as did her baseline mental status. This is the last EGK on file for this patient:
She experienced asystolic arrest and died the next day.
Discussion
Persistent ST-segment elevation following PCI has been shown to closely reflect the presence of microvascular reperfusion injury as demonstrated by impairment of microcirculatory flow on PET imaging and intracoronary contrast echocardiography. Mechanisms of reperfusion injury include neutrophil infiltration, tissue edema, and direct mivrovascular damage following tissue hypoxia. Impaired microcirculatory reperfusion resulting from these mechanisms has been correlated with more extensive infarction and a worse clinical outcome. Roughly 30%-40% of patients undergoing PCI demonstrate persistent STE on hospital discharge.
In their 1999 publication, Claeys et al. utilized persistent ST-segment elevation following PCI to identify patients at risk for reperfusion injury. They report, “Patients >55 years of age with systolic pressures <120mmHg were at high risk for development of impaired reperfusion compared with patients not meeting these criteria (72% versus 14%, P<0.001).” (Claeys et al., 1999, p.1972)
Also in 1999, Matetzky et al. demonstrated similar findings comparing clinical outcomes in patients with comparable angiographic results following PCI but contrasting presentations on ECG regarding the persistence of ST-segment elevation. Results of this research indicated that, “…ST segment elevation resolution was associated with better predischarge left ventricular ejection fraction…. Group B patients [those with persistent STE], as compared with those of Group A [those with early resolution of STE], had a higher incidence of in-hospital mortality (11% vs. 2%, p 0.088), congestive heart failure (CHF) (28% vs. 19%, odds ratio (OR) 4, 95% confidence interval (CI) 1 to 15, p 0.04), higher long-term mortality (OR 7.3, 95% CI 1.9 to 28, p 0.004 with Cox proportional hazard regression analysis) and long-term CHF rate (OR 6.5, 95% CI 1.3 to 33, p 0.016 with logistic regression).” (Matetzky et al., 1999, p.1932)
In 2007, Galiuto et al. investigated the clinical correlates found in patients demonstrating persistent STE following primary or rescue PCI. They report, “Such an ECG sign at hospital discharge may be considered associated with a large infarct size, and, in 30% of cases, with LV aneurysm formation and with continuing LV remodeling.” (Galiuto et al., 2007, p.1380)
These three studies reviewed between 100-150 patients each.
In 2010, however, Verouden et al. reviewed over 2100 patients undergoing PCI with the intent of further characterizing the clinical and demographic determinants of persistent ST-elevation after coronary intervention. They report that, “Incomplete ST-segment recovery was a strong predictor of long-term mortality…,” and, “…incomplete ST-segment recovery at the end of PCI occurred significantly more often in the presence of an age >60 years, nonsmoking, diabetes mellitus, left anterior descending coronary artery–related STEMI, multivessel disease, and preprocedural Thrombolysis In Myocardial Infarction grade 3 flow.” (Verouden et al., 2010, p.1692)
References
Claeys, M., et al. (1999). Determinants and prognostic implications of persistent ST-segment elevation after primary angioplasty for acute myocardial infarction: importance of microvascular reperfusion injury on clinical outcome. Circulation. 1999;99:1972-1977, doi: 10.1161/01.CIR.99.15.1972
Galiuto, L., et al. (2007). Functional and structural correlates of persistent ST elevation after acute myocardial infarction successfully treated by percutaneous coronary intervention. Heart. 2007; 93: 1376-1380, doi: 10.1136/hrt.2006.105320
Matetzky, S., et al. (1999). The significance of persistent ST elevation versus early resolution of ST segment elevation after primary PTCA. Journal of the American College of Cardiology. Vol. 34, No. 7, 1999.
Verouden, N., et al. (2010). Clinical and angiographic predictors of ST-segment recovery after primary percutaneous coronary intervention. American Journal of Cardiology. 2010;105:1692-1697.
Case No. 6: Competency of Junctional Escape Pacemakers in Wenckebach
In 1899, 4 years prior to the invention of the EKG, Karel Wenckebach utilized arterial kymographs to characterize a clinical presentation of regular irregularity in cardiac systoles. This entity was to become one of most well known eponyms in medicine– what we now know as the Wenckebach phenomenon.
With this case, I want to call attention not to the rhythm itself, but to an unusual feature seen here in conjunction with the Wenckebach: look closely at the 4th QRS complex– how do we know this is a junctional escape and not simply a grossly prolonged PR interval? If such a distinction can be made, what clinical significance should we ascribe to the finding?
In normal cardiac physiology, as the impulse descends from the His bundle, the first portion of myocardium to be activated is the septum; thus the initial deflection of the QRS complex reflects the vector of septal depolarization. Further, given that the septum is predominantly supplied by the posterior fascicle of the LBB, the septal myocardium depolarizes from left to right; it is therefore not pathological to note an initially negative deflection in lead II.
Graphic courtesy of http://www.cvphysiology.com/Arrhythmias/A016.htm
What is noteworthy about the complexes on these tracings, however, is not that there is an initial Q-wave in II, but that the escape complexes in the lead II tracing reveal a conspicuously more pronounced initial Q wave deflection than that present in the normative systoles. Although the QRS morphology and R-wave axis here remain consistent, pointing into the lower left quadrant (~+60°), in the escape complex there is a greater mass of initial electrical activity dissipating away from the lead II electrode (~–120°). This suggests that the ectopic focus responsible for these junctional escape beats is slightly more inferior relative to that authoring the normative QRS signals. In short, due to a more inferior position in the AV nodal tissue, a greater portion of myocardium would depolarize backwards towards the superior and rightward hexaxial quadrant, thus giving rise to the slightly greater Q wave in the escape complexes.
Although these observations on the initial deflection axis suffice to demonstrate a junctional rather than sinus origin to the complexes in question, it should be emphasized that alternative, “markedly slow pathways” through the junctional tissue may mimic this finding. Shinji Kinoshita et al. have recently presented an interesting and more sophisticated analysis of how to approach this issue in their article, Apparent AV Junctional Escape in Wenckebach AV Block: Markedly Slow Conduction Through The Slow AV Pathway, which can be found in the Journal of Cardiovascular Medicine 2009, 10:161–166.
Given that the existence of a junctional focus can be positively or negatively established, however, we might propose a risk stratification dichotomy between Wenckebach pts demonstrating a functional escape pacemaker (as seen above) and those with “escape failure” who must endure QRS dropping without junctional compensation. While systematic study and clinical correlates remain necessary to validate this hypothesis, pts in this latter category would presumably carry greater risk of symptomatic bradycardia than those in the former, and the absence of junctional escape activity might therefore be a positive predictor of greater morbidity in this population.
Case No. 4 A: Atypical Symptoms with a Critical Lesion
A 64 yr old white female presented to EMS with n/v/d times three days and a recent episode of orthostatic syncope. She had no complaints of chest discomfort or shortness of breath. She was pale in apearance and found to be hypotensive on exam.
Although AV dissociation can be appreciated in the first three tracings, the fourth appears consistent with undifferentiated 2nd degree conduction block and prolonged (>200 ms) PR interval with bigeminal junctional escape. Close examination of II and V4-6 reveal a subtle morphological variation in the complexes, supporting the argument for multiple depolarization foci. A narrow-complex junctional escape rhythm is typical of a culprit RCA lesion resulting in often transient ischemia to the superior portion of the nodal tissue. Wide-complex 3rd degree block more frequently reflects a significant LCA infarction which has resulted in distal, more inferior conduction system damage that is less likely to recover. Note that the ST elevation in III is greater than that in II— a finding that has been correlated with RV involvement, although I know of no EBM trials to support this. It is regrettable that right-sided and posterior leads were not recorded.
AV conduction abnormalities can be appreciated in as much as 30% of inferior wall MIs owing to the ~70% prevalence of the RCA as the dominant vessel supplying the AV nodal branch. Left dominant coronary systems present a variation to the predictability of progressive conduction pathway ischemia but constitute only 10% of the populace. This leaves 20% with co-dominant systems. In the absence of confounding factors, it would be interesting if there has been a study demonstrating a decreased incidence of AV conduction blocks in pts with redundant AV nodal circulation who present with acute coronary syndrome.
Despite what appear to be convalescent ECG changes over the course of these three 12-leads, the pt. deteriorated rapidly in the ED and required pressor support and intubation before she could be transported to the cath lab. The outcome is unknown.
Case No. 4 B: Chest Pain
A 68 year old white male presenting to EMS with chest pain and a history of HTN. No further on this case in known at this time.
This ECG demonstrates a 3:2 Wenkibach phenomenon with an initial inconsistency, possibly due to an A:V ratio shift or artifactual event. Note the subtle elevation in V6; again, a 15lead tracing would have been optimal here.
It should not be forgotten that although inferior wall STEMI typically results from RCA occlusion, it may also arise from a lesion in the Circumflex; in the latter case, the right heart is spared and RCA dependent conduction system elements remain unaffected. Dr. Smith has recently presented an example of this phenomenon as well as a review of a risk stratification ECG algorhythm recently proposed to deliniate RCA vs CLX lesions on ECG. While the case above meets several of these criteria (aVR depression and V6 eleveation), the manifest conduction system involvment all but eliminates the possibility of a CLX eitiology. I hope to post a better example of the DeVerna RCA/CLX decision tool in the near future; see Dr. Smith’s ECG site for a superior and more appropriately exemplified discussion of this new research.